low dose naltrexone half life
21 All the studies combined low-intensity lifestyle modification counseling with naltrexonebupropion or placebo except. If adrenal insufficiency is diagnosed.

Adding Ultralow Dose Naltrexone To Oxycodone Enhances And Prolongs Analgesia A Randomized Controlled Trial Of Oxytrex The Journal Of Pain
Once withdrawal symptoms start ramping up former steroid users experience decreased energy and alertness.

. In the developing world it is also used to treat skin sporotrichosis and phycomycosis. Low-dose naltrexone LDN has been demonstrated to reduce symptom severity in conditions such as fibromyalgia Crohns disease multiple sclerosis and complex regional pain syndrome. Symptoms start faintly and may include irritability headache and nausea.
8 79 9 80 A single 50 mg oral dose of naltrexone has been found to block brain MORs and opioid effects for at least 48 to 72 hours. Alternatively dose equivalent to one-third of patients calculated 24-hr PO morphine requirement q8hr. As a medication it is used to treat hyperthyroidism in radiation emergencies and to protect the thyroid gland when certain types of radiopharmaceuticals are used.
The first symptoms of withdrawal begin at some point within five days of the users last dose depending on the half-life of the steroid. Dosing Forms Strengths. Symptoms may include nausea vomiting anorexia fatigue weakness dizziness and low blood pressure.
As a supplement it is used in those who have low. See what others have said about Contrave Bupropion And Naltrexone including the effectiveness ease of use and side effects. MS Contin dose equivalent to one-half of patients calculated 24-hr PO morphine requirement q12hr.
But it has greater oral bioavailability and a longer biologic half-life. The half-life of occupancy of the brain MOR and duration of clinical effect of naltrexone are much longer than suggested by its plasma elimination half-life. Contrave Bupropion And Naltrexone received an overall rating of 6 out of 10 stars from 114 reviews.
For use as adjunct to reduced-calorie diet and increased physical activity for long-term weight management in adults with initial body mass index of 30 kgm² obese or 27 kgm² overweight in presence of at least 1 weight-related comorbidity eg hypertension type 2. The COR-Diabetes study evaluated overweight and obese patients with type-2 diabetes. Naltrexone HCl was approved by FDA in 1984 for the treatment of opioid addiction.
Potassium iodide is a chemical compound medication and dietary supplement. 24 COR-I was the only study of the four that compared naltrexonebupropion 16 mg360 mg to naltrexonebupropion 32 mg360 mg and to placebo.

A Summary Of Clinical Experience On Low Dose Naltrexone Ldn In Download Table

Naltrexone Naltrexone Bupropion Combination Mechanism Of Action

Naltrexone Naltrexone Bupropion Combination Mechanism Of Action
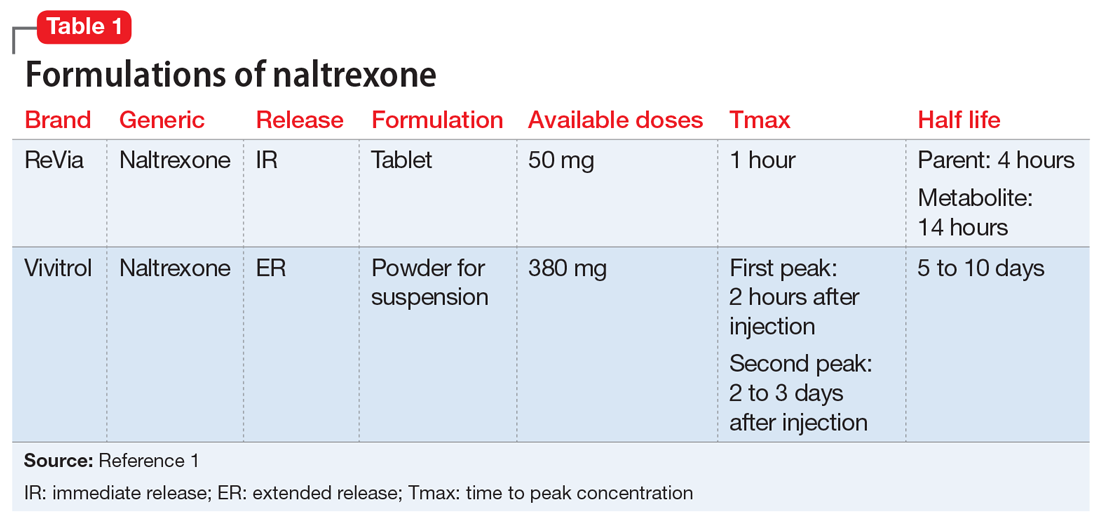
Managing Procedural Pain In A Patient Taking Naltrexone Mdedge Psychiatry
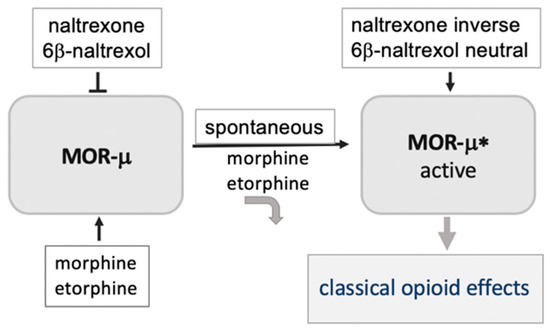
Molecules Free Full Text Biased Opioid Antagonists As Modulators Of Opioid Dependence Opportunities To Improve Pain Therapy And Opioid Use Management Html

A Summary Of Clinical Experience On Ultra Low Dose Naloxone Naltrexone Download Scientific Diagram
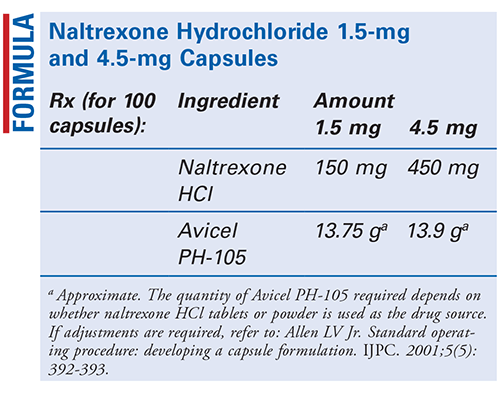
Naltrexone Hydrochloride 1 5 Mg And 4 5 Mg Capsules

Medication Assisted Therapies For Opioid Use Disorders In Patients With Chronic Pain Journal Of The Neurological Sciences

A Summary Of Clinical Experience On Low Dose Naltrexone Ldn In Download Table

Low Dose Naltrexone Ldn Pain Spa
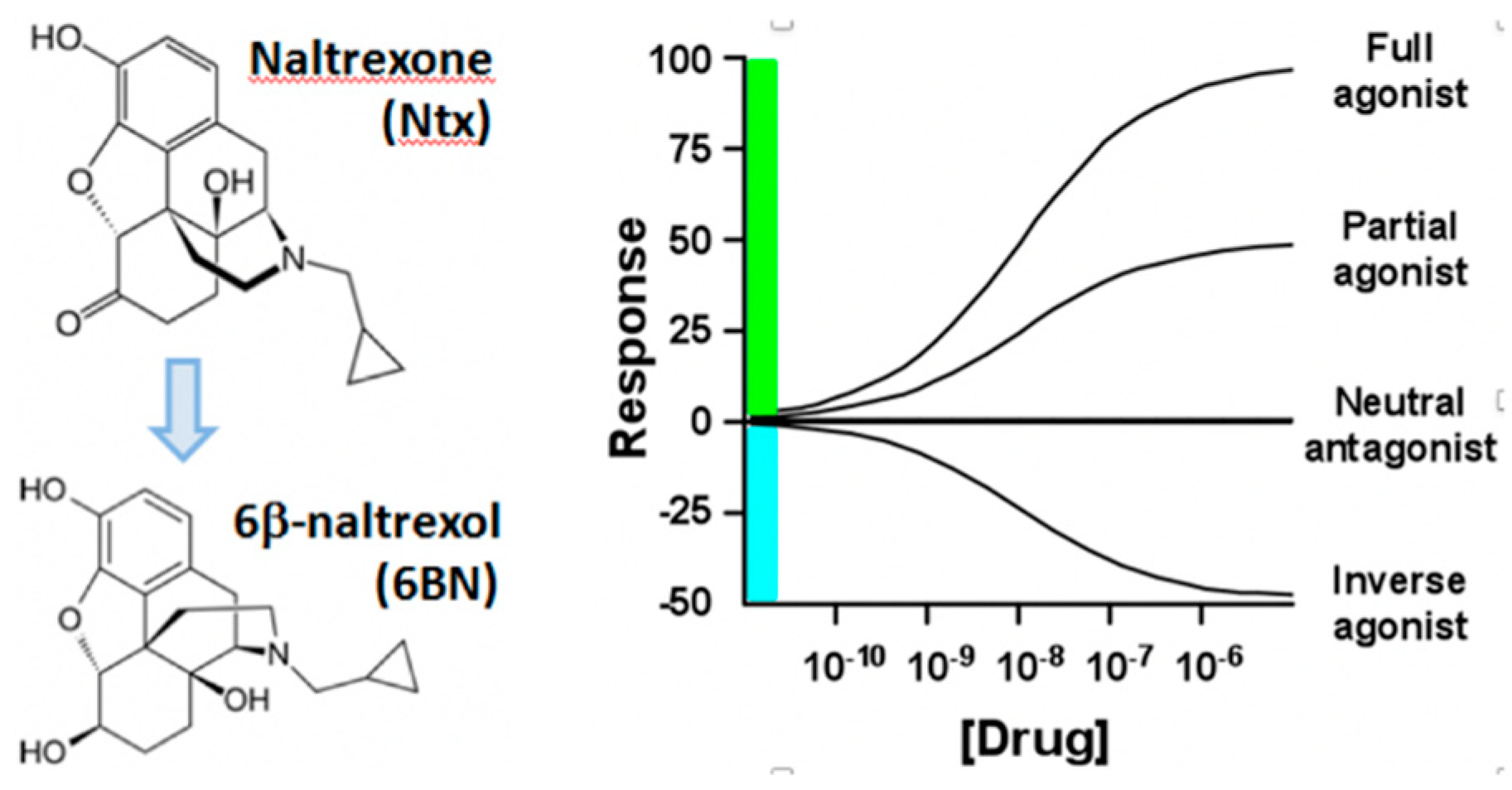
Molecules Free Full Text Biased Opioid Antagonists As Modulators Of Opioid Dependence Opportunities To Improve Pain Therapy And Opioid Use Management Html

A Summary Of Clinical Experience On Low Dose Naltrexone In Crohn S Download Table

Naltrexone Naltrexone Bupropion Combination Mechanism Of Action

Pharmacology In Alcohol Use Disorder Naltrexone Acamprosate And Disulfiram Psychopharmacology Institute

Low Dose Naltrexone In Dermatology Jddonline Journal Of Drugs In Dermatology

Naltrexone Naltrexone Bupropion Combination Mechanism Of Action